NEWS & EVENTS
Clinical Therapy & Healthcare
European Marketing Authorization for leukemia CAR-T therapy
Following encouraging developments, the EC has granted marketing authorization for AUCATZYL®, an autologous CD19 CAR T-cell therapy developed by Autolus Therapeutics. AUCATZYL was recently assessed in an open-label, multi-center, single arm study, FELIX, where patients exhibited high and durable response to the therapy.
The authorization allows the therapy to be used for the treatment of adult patients with r/r B-ALL, in 27 European Union Member States, Iceland, Norway and Liechtenstein.
“We believe AUCATZYL represents an important new treatment option for physicians treating adult r/r B-ALL patients. With the EU marketing authorization, we are now evaluating market entry opportunities in EU countries,” said Christian Itin, CEO of Autolus.
Currently, AUCATZYL is approved by the FDA and authorized by the UK Medicines and Healthcare products Regulatory Agency (London, UK).
C


Updated 2025 List of FDA-Approved Cell and Gene Therapies
By Professor Paul Knoepfler, Ph.D. / 7 Comments / January 21, 2025 / FDA Approved Cell and Gene Therapies, FDA-approved stem cells / FDA approved stem cell therapies, Informational
What are the current FDA-approved cell and gene therapies? How many of those involve true stem cells?
Many of us have wished for a more specific list of FDA-approved stem cell therapies. Patients and fellow scientists often asked me about such lists. They even wish for annotated lists.
For that reason, I’ve made and kept updated a comprehensive list of FDA Approved Cell and Gene Therapies, which is the focus of today’s post. These include FDA-approved stem cell therapies. I’ve updated the list now in 2025 to include Casgevy and Lyfgenia, approved cell and gene therapies for sickle cell.
“Stem cell products are regulated by FDA, and, generally, all stem cell products require FDA approval. Currently, the only stem cell products that are FDA-approved for use in the United States consist of blood-forming stem cells (also known as hematopoietic progenitor cells) that are derived from umbilical cord blood. These products are approved for use in patients with disorders that affect the production of blood (i.e., the “hematopoietic” system) but they are not approved for other uses.”
After getting Vertex’s Zimislecel most appear cured of type 1 diabetes in small trial
By Professor Paul Knoepfler, Ph.D. / 4 Comments / June 21, 2025 / stem cells for diabetes, Vertex diabetes, Zimislecel / diabetes
In groundbreaking results, Vertex Pharmaceuticals presented and published results showing that ten out of twelve patients in a small trial exhibited signs of a cure for their type 1 diabetes after receiving the stem cell therapy Zimislecel.
This is extremely exciting. While still early days for this therapy and there are some caveats (discussed below), my sense is that this therapy is likely on track to gain FDA approval in 2026.All participants:
“Demonstrated engraftment with glucose-responsive endogenous C-peptide production, which was durable through one year of follow-up.
Achieved the ADA targets of HbA1c <7% and time in range of >70%.
Were free of SHEs from day 90 onwards.
Had a reduction in exogenous insulin use (mean reduction in daily insulin dose: 92%).
10/12 (83%) no longer required exogenous insulin at Month 12.
Achieved the Phase 1/2 primary endpoint of elimination of SHEs with HbA1c <7%.”
These are impressive efficacy outcomes so far.


CLEVELAND, April 29, 2025 (GLOBE NEWSWIRE) -- Abeona Therapeutics Inc. (Nasdaq: ABEO) today announced the U.S. Food and Drug Administration (FDA) has approved ZEVASKYN™ (pronounced as ‘ZEE-vah-skin’) (prademagene zamikeracel) gene-modified cellular sheets, also known as pz-cel, as the first and only autologous cell-based gene therapy for the treatment of wounds in adult and pediatric patients with recessive dystrophic epidermolysis bullosa (RDEB), a serious and debilitating genetic skin disease. There is no cure for RDEB and ZEVASKYN is the only FDA-approved product to treat RDEB wounds with a single application.
“Today’s approval of ZEVASKYN represents a pivotal moment in the treatment of RDEB, answering the call of people living with the clinical, economic, and human impact of this devastating disease,” said Vish Seshadri, Ph.D., M.B.A., Chief Executive Officer of Abeona. “We have heard from the RDEB community that there is a persistent unmet need to reliably address RDEB wounds, especially those that are chronic and prone to infection. Through a single surgical application, ZEVASKYN can now offer people with RDEB the opportunity for wound healing and pain reduction in even the most severe wounds, as evidenced by the results from our pivotal Phase 3 study.”
First-of-its-kind gene therapy with robust body of clinical evidence
The FDA approval of ZEVASKYN is based on the pivotal Phase 3 VIITAL™ study (NCT04227106), a multi-center, randomized, intrapatient-controlled trial that met its two co-primary efficacy endpoints demonstrating statistically significant healing of 50 percent or more from baseline in large chronic RDEB wounds, and pain reduction from baseline as assessed by the Wong-Baker FACES scale, as evaluated at six months after treatment.
Across 43 large and chronic wounds treated with a single application of ZEVASKYN, 81 percent of wounds showed 50 percent or more healing (P<0.0001) as evaluated at six months, compared to 16 percent in 43 matched control wounds treated with standard of care. The most common adverse events were observed in fewer than five percent of patients and included procedural pain and itch.
Stem Cell Discovery in Human Retina May Lead to Retinal Degeneration Treatments
Mice that received transplants of a newly discovered retinal stem cell population showed improved vision.
Apr 3, 2025| 2 min read
For individuals with inherited eye disorders such as retinitis pigmentosa or macular degeneration, the irreversible degeneration of the retina—a layer of photoreceptors at the back of the eye—can cause impaired vision and lead to blindness. Although millions of people worldwide suffer from retinal degeneration-associated blindness, no curative treatment is available.1
Stem cell therapies hold promise for promoting regeneration. Retinal stem cells (RSCs) possess self-renewal capabilities and can continuously generate new cells. While lower vertebrates like zebrafish and amphibians have RSCs, their existence in mammals has remained uncertain.
In a study published in Science Translational Medicine, scientists discovered a stem cell population in the retina of human fetuses, which they called human neural retinal stem-like cells (hNRSCs).2 The team also found that a common retinal organoid model contains a population of hNRSCs with a similar transcriptional profile. When researchers transplanted the organoid-derived cells into a mouse model of retinitis pigmentosa, the treatment alleviated retinal degeneration and improved visual function. These findings provide a promising new approach for restoring vision in individuals with retinal diseases.
In the present study, Jianzhong Su and his colleagues at Wenzhou Medical University used single-cell and spatial transcriptomic sequencing to characterize gene expression in human retinal fetal samples that were donated following a terminated pregnancy. In doing so, they identified a distinct population of cells that could self-renew and develop into various cell types.
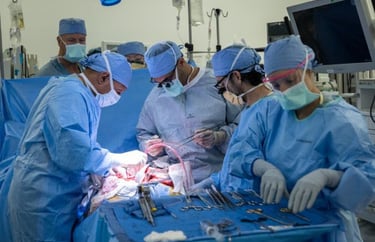
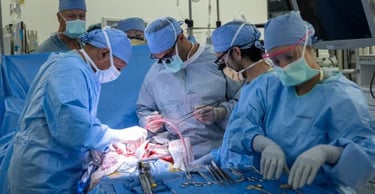
World-first bladder transplant a success
Oscar Larrainzar, a 41-year-old father of four, has made history by becoming the first person to undergo a bladder transplant. Larrainzar had been on dialysis after most of his bladder and both kidneys were removed to treat cancer and end-stage kidney disease. Following the eight-hour surgery, the transplanted kidney immediately made a large volume of urine. “It’s the first time he has been able to pee in seven years,” said urologic surgeon Inderbir Gill. “For all of us, this is huge.”
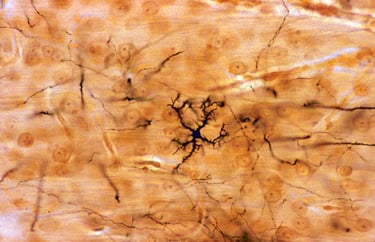

How CAR-T therapy can harm the brain
People with cancer who undergo CAR-T-cell therapy often report lingering cognitive symptoms, such as difficulty with attention and memory. A study has now shown that mice develop inflammation in the brain and deficits in attention and short-term memory after being given CAR-T-cell therapy. Similar changes were seen in postmortem brain tissue of four people with cancer given the treatment.
Nature Research Highlight | 1 min read
Reference:Cellpaper(12 May)
As


Mytos Announces Manufacturing Collaboration with Aspen Neuroscience in Parkinson
January 08, 2025 09:00 AM Eastern Standard Time
LONDON--(BUSINESS WIRE)--Mytos, an innovative leader in automated cell manufacturing, has entered into a manufacturing collaboration with Aspen Neuroscience, Inc. to automate the manufacturing of autologous dopaminergic neuronal precursor cells (DANPCs) for ANPD001, Aspen’s investigational cell therapy program for Parkinson’s disease (PD).
“Aspen’s therapies hold transformative potential for millions of people living with Parkinson’s. By integrating Mytos’ iDEM technology, Aspen can accelerate the scale-up of its ANPD001 investigational therapy, bringing it to more patients.”
The DANPCs, designed to replace lost dopamine-producing neurons in the brain, are differentiated from induced pluripotent stem cells (iPSCs), which are derived from the patient’s own skin cells. This approach holds the promise of restoring lost function without the need for immunosuppressive drugs.
As part of the partnership agreement, Aspen will integrate Mytos’ iDEM automated cell culture technology into its new manufacturing facility in Torrey Pines, Calif. The iDEM automated cell culture technology combines fluidics, advanced imaging, and mechanical movement and control to enable fully hands-off production of specific human cell types. This agreement represents the potential for substantial payments to Mytos over the coming years.
Cell NEWS
04 February 2025
The science behind the first pig-organ transplant trial in humans
The small trial will help to establish whether kidneys from genetically modified pigs can be transplanted into people safely and effectively.
The first clinical trial testing whether pig kidneys can be safely transplanted into living people has been approved by the US Food and Drug Administration (FDA). As part of the trial, which will begin later this year, kidneys from genetically modified pigs will be transplanted into people with chronic kidney disease whose organs no longer function independently.
The FDA’s green light will bring the experimental procedure a step closer to one day supplying organs to the thousands of people who are waiting for a donor organ. “The start of formal clinical trials is very exciting,” says Jay Fishman, a specialist in transplant infectious diseases at Massachusetts General Hospital in Boston.
About half a dozen people in the United States and China have received organs from genome-edited pigs — kidneys, hearts, liver and a thymus — but the surgeries were approved on compassionate grounds, meaning the patients were very sick and had no other options. Most recipients did not survive beyond a few months after the transplants for various reasons, including that they were too sick to cope with major surgery.
Nov 13, 2024
Clinical trial results are sho
Weekly reads: RFK Jr. stem cell summit, FDA OKs eye cell therapy, cartilage repair
By Professor Paul Knoepfler, Ph.D. / 2 Comments / March 16, 2025 / RFK Jr. stem cells, RFK regenerative medicine / stem cell politics
I had a feeling late last year that I’d frequently be writing about RFK Jr. and his impact on the FDA. However, I didn’t realize some of that would be writing for STAT News.
As I mentioned a few days ago, I now have a new regular column over there. I thought the column would be just a once-a-month kind of thing, but so much has been happening that I put together another column this week.
Good news: FDA approves new cell therapy for rare eye condition
FDA approves Neurotech cell therapy to slow a rare degenerative eye disease, Endpoints. This is exciting news. The FDA doesn’t often approve cell therapies. Here’s the company’s announcement: “Neurotech’s ENCELTOTM (revakinagene taroretcel-lwey) Approved by the FDA for the Treatment of Macular Telangiectasia Type 2 (MacTel).” Check out my updated comprehensive, lightly-annotated list of FDA approvals of cell and gene therapies.
This other piece from Endpoints is also interesting: FDA’s medical review departments remain untouched (for now) by Musk’s cuts. I’m not sure this “untouched” status will continue and as I’ve written before, we’ve already seen some key departures from CBER.


CAR T-Cell Therapy Shows Efficacy in Young Patients with Incurable Brain Cancer
Nov 13, 2024
Clinical trial results are showing the first successes against solid tumors for CAR-T cells. The findings offer hope for children with a group of deadly brain and spinal cord tumors, including a cancer called diffuse intrinsic pontine glioma, or DIPG. The immune-cell therapy shrank children’s brain tumors, restored neurologic function and—for one participant—erased all detectable traces of a brain cancer typically considered incurable.
The findings are published in Nature in the paper, “Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas.”
“This is a universally lethal disease for which we’ve found a therapy that can cause meaningful tumor regressions and clinical improvements,” said Michelle Monje, MD, PhD, professor of neurology at Stanford Medicine. “While there is still a long way to go to figure out how to optimize this for every patient, it’s very exciting that one patient had a complete response. I’m hopeful he has been cured.” The patient, 20-year-old Drew, wants his outcome to be the first of many. “I’m hoping they’ll learn from all my successes to help other kids,” he said.
Diffuse midline gliomas, which can grow in the brain or spinal cord, are diagnosed in a few hundred children and young adults in the United States annually and have a median survival time of about a year. Radiation therapy offers only temporary relief, no effective chemotherapy drugs exist, and the tumors cannot be surgically removed. DIPG, the subtype of disease that occurs in the brainstem, has a five-year survival rate below 1%.
The type of CAR-T cell therapy used in the trial was developed at Stanford Medicine: In 2018, Monje’s team discovered that DIPG and other diffuse midline glioma tumor cells produce a large amount of the surface marker GD2. Mackall’s team had already engineered CAR-T cells to target GD2, which is found in other cancers. The researchers showed that GD2-targeting CAR-T cells eradicated DIPG tumors in animal models.
Cell therapy weekly: FDA approves CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia
14 Nov 2024
Written by Kadeja Johnson (Assistant Digital Editor)
This week: Terumo Blood and Cell Technologies (Terumo BCT; CO, USA) has launched a new business unit to improve patient care throughout the treatment journey. Plus, the US Food and Drug Administration (FDA; MD, USA) has approved a gene therapy for aromatic L-amino acid decarboxylase (AADC) deficiency and a CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL).
The FDA has approved KEBILIDI™ (eladocagene exuparvovec-tneq), a recombinant adeno-associated virus serotype 2 (rAAV2)-based gene therapy developed by PTC Therapeutics (NJ, USA) for treating AADC deficiency. This marks the first gene therapy approved in the US that is directly administered to the brain.
AADC deficiency is a rare, life-threatening genetic disorder that impairs dopamine production. KEBILIDI aims to deliver a functional DDC gene directly into the putamen, thereby increasing the AADC enzyme to restore dopamine synthesis. The therapy received accelerated approval based on safety and efficacy results from an ongoing global clinical trial, which demonstrated de novo dopamine production and improvements in motor development milestones.
The news highlights:
FDA News Release
FDA Approves First Mesenchymal Stromal Cell Therapy to Treat Steroid-refractory Acute Graft-versus-host Disease
For Immediate Release:
December 18, 2024
Today, the U.S. Food and Drug Administration approved Ryoncil (remestemcel-L-rknd), an allogeneic (donor) bone marrow-derived mesenchymal stromal cell (MSC) therapy indicated for the treatment of steroid-refractory acute graft-versus-host disease (SR-aGVHD) in pediatric patients 2 months of age and older.
Ryoncil is the first FDA-approved MSC therapy. It contains MSCs, which are a type of cell that can have various roles in the body and can differentiate into multiple other types of cells. These MSCs are isolated from the bone marrow of healthy adult human donors.
“Today’s decision marks an important milestone in the use of innovative cell-based therapies to treat life-threatening diseases with devastating impacts on patients, including children,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research (CBER). “This first mesenchymal stromal cell therapy approval demonstrates the FDA’s commitment to supporting the development of safe and effective products that could improve the quality of life for patients with symptoms that are unresponsive to other therapies.”
February 5, 2024
26 September 2024
Stem cells reverse woman’s diabetes — a world first
She is the first person with type 1 diabetes to receive this kind of transplant.
Diabetes reversed with stem cells
A woman with type 1 diabetes started producing her own insulin less than three months after a transplant of reprogrammed stem cells. This case represents the first successful treatment for the disease using stem cells from the recipient’s own body, which could avoid the need for immunosuppressants. She was injected with the equivalent of 1.5 million stem-cell-derived islets in June 2023. While promising, the woman’s cells must continue to produce insulin for up to five years before considering her ‘cured’, cautions endocrinologist Jay Skyler.
Nature | 6 min read
Reference: Cell paper
Human Stem Cells Repair Retinas in Monkey Models of Macular Holes
Researchers from RIKEN and elsewhere have published the results of experiments that assessed whether transplanting human stem-cell retinal organoid sheets could effectively treat macular holes in monkeys. They saw evidence of graft survival, development of light-detecting retinal cells, as well as improved eye fixation and response to light. + MORE
Their findings are described in a paper published in Stem Cell Reports titled, “Transplantation of human pluripotent stem cell-derived retinal sheet in a primate model of macular hole.”
https://www.bayer.com › cell-therap...
27 sept. 2024 — Pharmaceuticals
BlueRock Therapeutics’ investigational cell therapy bemdaneprocel for Parkinson’s disease shows positive data at 24-months
The safety profile at 24 months is consistent with earlier findings, demonstrating that bemdaneprocel continues to be well-tolerated by patients ...
About bemdaneprocel (BRT-DA01) and the exPDite Phase 1 trial Bemdaneprocel (BRT-DA01) is an investigational cell therapy designed to replace the dopamine producing neurons that are lost in Parkinson’s disease. These dopaminergic neuron precursors are derived from pluripotent stem cells that are human embryonic stem cells. In a surgical procedure, these neuron precursors are implanted into the brain of a person with Parkinson’s disease. When transplanted, they have the potential to reform neural networks that have been severely affected by Parkinson’s and to restore motor and non-motor function to patients.
IND clearance for bispecific T-cell therapy for glioblastoma
September 04, 2024 08:00 AM Eastern Daylight Time
The US FDA has cleared an IND application for Adaptin Bio’s (NC, USA) Brain Bispecific T-cell Engager (BRiTE) therapy for glioblastoma, APTN-101.
APTN-101 has been developed to precisely target EGFRvIII proteins associated with brain tumors and eliminate the glioma cells. In preclinical studies, APTN-101 demonstrated potent elimination of malignant glioma tumors across various aggressive orthotopic models. Adaptin plans to evaluate the safety and efficacy in a Phase I clinical trial in patients with Grade IV malignant glioma.
“Our proprietary BRiTE technology harnesses the immune system’s remarkable ability to target and deliver therapeutics to specific tissues, including the brain, potentially revolutionizing treatment for difficult-to-treat cancers,” commented Adaptin Bio’s CEO, Michael J Roberts. “APTN-101 validates the BRiTE platform and its ability to enhance transfer of therapeutics into the brain. We are committed to advancing this novel therapy as a new potential therapy for glioblastoma patients who desperately need new therapies.H
Service titleThe Web's Daily Resource for Parkinson's News
In the 09/11/2024 edition:
FDA clears brain mapping tool for neuromodulation therapies
by Lindsey Shapiro, PhD | September 11, 2024
The U.S. Food and Drug Administration (FDA) has cleared Turing Medical’s automated brain mapping technology designed to help doctors develop personalized plans for deep brain stimulation (DBS) and other neuromodulation therapies in people with neurological conditions such as Parkinson’s disease.
Called Bullsai Identify, the platform uses artificial intelligence algorithms to analyze advanced imaging data and create a detailed map of a person’s brain. Doctors can then use those maps to find the optimal target for that specific person’s neuromodulation treatment.
Biobanking
First Engineered T Cell Therapy for Solid Tumors Approved by the FDA
By IPM staff August 5, 2024
Share
The immunotherapy afamitresgene autoleucel (afami-cel) for the treatment of adults with the rare soft tissue cancer called synovial sarcoma was granted accelerated approval late last week by the U.S. Food and Drug Administration (FDA). Afami-cel is the first engineered T cell therapy to receive FDA approval for treatment of a solid tumor.
“This treatment offers an important new option for people with this rare cancer,” said Sandra D’Angelo, MD, a sarcoma specialist who led the clinical at Memorial Sloan Kettering Cancer Center (MSK). “It is also an important step forward in the development of T cell therapies for solid tumors, which has been a major challenge.”
Afami-cel is not a CAR T therapy, rather it is a T cell receptor (TCR) therapy. Related to CAR Ts, TCRs can target proteins within the cell itself, while CAR T therapies only target proteins on the cell surface. The cell engineering process for afami-cel, however, is virtually the same as for CAR T cell therapies: a patient’s T cells are collected from their blood, then engineered to recognize and fight a specific target in cancer cells, which are then reinfused into the patient.
The target for afami-cel is a a protein called MAGE-A4.
The FDA approval for afami-cel was based on results from Adaptimmune’s pivotal SPEARHEAD-1 trial for treating metastatic synovial sarcoma and myxoid round cell liposarcoma (MRCLS), results of which were published in April in The Lancet. MRCLS is a distinct disease that shares clinical and biological features with synovial sarcoma including the expression of the MAGE-A4 cancer antigen.
In total, 52 patients were treated with afami-cel (44 with synovial sarcoma, eight with myxoid round cell liposarcoma). All patients had not responded to earlier lines of therapy. Data from the trial showed that 37% of the patients had their tumors shrink after receiving a single dose. Efficacy rates were 39% for synovial sarcoma patients and 25% in those with MRCLS.
Synovial sarcoma patients responded to their treatment for an average of 11.6 months; MRCLS patients for an average of 4.2 months. D’Angelo, who was the lead author the The Lancet piece noted that these responses were important for this group of patients as many had exhausted all other treatment options.
Prior to receiving the therapy, all patients enrolled in the trial received chemotherapy. Hematologic toxicities were the most common adverse events, which the investigators expected as a result of lymphodepletion which commonly occurs in chemotherapy patients. Cytokine release syndrome (CRS), a potentially serious side effect, was experienced by 71% of trial participants, though symptoms of CRS were not severe for most patients.
Regeneration Medicine Insurance
HIV-Stem cell therapy
Second HIV Patient "Cured" with Stem Cell Transplant at Charite, Using Slightly Different Genetic Recipe
After transplant, the patient has had no detectable virus for more than five years even though he is not taking antiviral medications. Why this patient was cured while the virus has resumed replicating in comparable cases is still unclear. This new case will be presented at the International AIDS Conference in Munich on July 24. + MORE
https://insightplus.mja.com.au › a-new-japanese-stem-cell...
The Japanese health ministry approved Stemirac last December, and the treatment is now available to the Japanese public, with most of the $140,000 cost covered by the country's universal National Health Insurance.
So far, only three treatments have earned conditional approval, but the government aims to add to the list. Japan’s economic development ministry has estimated that regenerative medicine will be a $10 billion market in the country by 2030.a short text about your service. Highlight key benefits for potential clients.
World-First CAR T-Cell Therapy for Child With Lupus NEWS
World-First CAR T-Cell Therapy for Child With Lupus
CAR T-cell therapy has been used for the first time to treat lupus in a child, restoring the patient's ability to live a normal life.
Published: July 9, 2024 Original story from FAU
“One year after the treatment, I feel as good as I did before my diagnosis, except for a few colds,” says Uresa A. today. In June 2023, Uresa (who was 15 years old at the time) received CAR-T cells at Universitätsklinikum Erlangen. The treatment was the last resort to slow down systemic lupus erythematosus (SLE), which is a serious autoimmune disease that was attacking Uresa’s body and suddenly and seriously affected her ability to lead a normal life.
Treatment saves 15-year old patient with autoimmune disease SLE
This procedure was unusual since CAR-T cells had previously only been used for leukemia or lymphoma and during studies on adults with certain advanced autoimmune diseases. These cells had previously never been used for treating children with autoimmune diseases. The girl, who is now 16 years old, was the first child with SLE to receive the immune therapy as part of an individual treatment attempt at Deutsches Zentrum Immuntherapie (DZI) at Universitätsklinikum Erlangen. The treatment team in Erlangen has now published the results of this successful treatment in the medical journal “The Lancet”.
“Simply the fact that we have administered CAR-T cells for an autoimmune disease is something special, since it was previously only authorized for certain advanced types of cancer,” explains Dr. Tobias Krickau, Uresa’s pediatric rheumatologist at the Department of Pediatrics and Adolescent Medicine (director Prof. Dr. Joachim Wölfle) at Universitätsklinikum Erlangen. “As Uresa’s SLE was progressing at a increasingly fast pace despite all her medication, we were confronted with the question of whether we should give these immune cells modified in a lab to a child. This off-label treatment for an autoimmune disease in a child had never been attempted anywhere in the world, as far as we knew,” says senior physician Krickau. This treatment involves removing some immune cells (T cells) from the patient before the CAR-T cell infusion and equipping these cells with a chimeric antigen receptor (CAR) in a special cleanroom laboratory. These CAR-T cells are then given to patients and dock onto damaging autoreactive B cells in their blood and destroy them. This results in a “reboot” for the immune system.
Uresa’s symptoms began in fall 2022 with migraines, exhaustion, joint and muscle pain and the red butterfly rash on the face that is typical for lupus. She had a high temperature, not enough red blood pigment and decreased levels of certain proteins that play a role in immune response and higher levels of lupus-specific autoantibodies that were attacking her healthy tissue. The SLE diagnosis was confirmed in an external clinic. Several treatments with various medications followed, however they attacked Uresa’s liver. Despite intensive treatments, her condition deteriorated and her kidney levels also worsened. A kidney condition known as lupus nephritis occurs in over 50 percent of patients with SLE. Although SLE is not as common in children as is is in adults, the disease is often more aggressive in children. Currently available treatments are more often associated with complications and serious side effects.
Dr. Krickau, who is a pediatric rheumatologist, ultimately took Uresa on as a patient at the end of 2022. “We started with the tablets and monthly intravenous therapies approved for children with the aim of suppressing her overactive immune system,” he explains. “But her kidney function rapidly deteriorated. Her kidneys were unable to transport fluids out of her body and Uresa developed severe water retention that caused her legs, hands, feet and face to swell up. She also developed high blood pressure.” From March 2023, she spent more time in hospital than at home. “In conjunction with the Pediatric Nephrology department, the next thing we tried was a highly immune-suppressive type of chemotherapy that can help with acute immunological kidney disease, but her condition did not improve,” says Tobias Krickau. In the space of a few months, doctors had to watch how the “lupus got out of control”, as Dr. Krickau says. “Our patient had an enormous amount of inflammatory messenger substances in her body. We tried flushing out the damaging autoantibodies from her blood with plasmapheresis – every day for two weeks. But Uresa’s kidneys continued to deteriorate until they finally failed completely and she had to start dialysis. Uresa, who is from Upper Franconia, was in hospital on a permanent basis by this point, isolated from friends and family, which affected her badly. “I don’t like hospitals, I just didn’t want to be there any more,” she remembers.
“I have nothing left to give her”
Dr. Krickau had come to the end of the line as far as treatments options were concerned. “I nothing left to give her” – this is what Dr. Krickau said to the team at the Pediatric Oncology department when he approached them with the idea of trying CAR-T cell therapy. “Up to now, this type of immune therapy has only been used in children with cancer and there is no experience with using it to treat autoimmune diseases at this young age. This is why such an initial application requires a particularly high amount of preparation and risk assessment,” explains Prof. Dr. Markus Metzler, head of Pediatric Oncology at Universitätsklinikum Erlangen. In addition, a great many organizational and legal obstacles had to be overcome. The Department of Medicine 5 – Hematology and Oncology (Director: Prof. Dr. Andreas Mackensen) at Universitätsklinikum Erlangen has a cleanroom laboratory for producing CAR-T cells as part of clinical studies and individual treatments. After discussing the case in detail with his colleagues, Prof. Mackensen agreed to produce and use CAR-T cells in the young patient. “The entire treatment team organized it all alongside their regular duties at the hospital in a very short amount of time,” emphasizes Dr. Krickau. “We initiated the CAR-T cell therapy for Uresa as part of an extended access program for severely ill patients in accordance with the German Medicinal Products Act and the Ordinance on Medicinal Products for Compassionate Use as something known as an individual treatment attempt.”
“Mum, you need to sign this!
Since 2021, a team led by Prof. Dr. Georg Schett, Director of the Department of Medicine 3 – Rheumatology and Immunology and Co-Speaker of the DZI, and Prof. Mackensen have successfully treated patients with various autoimmune diseases (including SLE) using CAR-T cells. In February 2024, the team presented 15 cases as part of a pilot study in the New England Journal of Medicine (DOI: 10.1056/NEJMoa2308917); the CASTLE study with 24 participants is currently ongoing. All those who received treatment are well – they are either healthy or have significantly fewer symptoms. This is ultimately what led the team to agree to treat Uresa. “PD Dr. Fabian Müller, senior physician at the Department of Medicine 5 and Prof. Schett explained to us how effective the treatment is in adults and how they thought it had potential for Uresa,” says her mother Albana A. “But I was scared of losing her. She convinced me herself and said ‘I want to do this, you have to sign the form!’ The whole team worked really hard and did everything for my daughter,” she remembers.
No previous treatment had been as effective
Uresa was given a mild course of chemotherapy to prepare for the treatment that made some space for the CAR-T cells in her blood at the BMT ward of the Department of Pediatrics and Adolescent Medicine. “The trick was to strike a balance between ensuring that the chemotherapy was working properly and was not being immediately flushed out by the dialysis on one hand, and making sure that the remaining kidney function was not being put at risk on the other,” explains pediatric oncologist Prof. Metzler. The big day came on June 26, 2023: Uresa was transferred from the pediatric oncology department to the Department of Medicine 5 and received the CAR-T cells specially modified for her. “Her kidney and lupus values improved from the third week after the treatment, “ says Dr. Krickau. “We had never achieved that with any medication previously.” All her symptoms gradually disappeared. At the end of July 2023, Uresa was finally able to go home and she completed her school leaving certificate. Her aim is to complete an apprenticeship in the automotive trade, move out of home soon, and get a dog. She is very happy that she can meet up with friends again, go out and lead the life of a normal teenager.
“There are still a large number of the CAR-T cells in her blood. Because they have not only eliminated damaging B cells, but also healthy ones, they are now missing, which means Uresa’s body can’t defend itself properly against certain infections,” explains Prof. Mackensen. Until her own B cells return Uresa therefore receives vital antibodies intravenously every four weeks at Universitätsklinikum Erlangen. “We were able to prevent any permanent damage to her organs because we decided to carry out the CAR-T cell therapy so early on,” says Dr. Krickau. “This success was only possible because of the way in which the physicians from several disciplines at Deutsche Zentrum Immuntherapie work closely together,” emphasizes Prof. Schett. Apart from the monthly IV of immunoglobulin, Uresa no longer needs any medication or dialysis. “She was seriously ill and her kidneys have now fully recovered, something nobody believed could happen to this extent,” says Dr. Krickau. In conjunction with the pediatric oncology department, pediatric rheumatologist Krickau is now planning a study with other children and teenagers with automimmune diseases where he hopes to use the great potential of CAR-T cells in pediatric rheumatology.
Reference: Krickau T, Naumann-Bartsch N, Aigner M, et al. CAR T-cell therapy rescues adolescent with rapidly progressive lupus nephritis from haemodialysis. The Lancet. 2024;403(10437):1627-1630. doi: 10.1016/S0140-6736(24)00424-0
Stem Cell-Derived Therapy Shows Early Promise for Resistant Liver Cancer NEWS
Stem Cell-Derived Therapy Shows Early Promise for Resistant Liver Cancer
An innovative new stem cell-derived therapy could be used to better target and treat the most common form of liver cancer.
Published: July 10, 2024
| Original story from The University of California San Diego
Researchers at University of California San Diego have found that the most common form of liver cancer — one with a high mortality rate — can be better targeted and treated using an innovative new stem cell-derived therapy, according to a recently published study in Cell Stem Cell
The treatment, not yet studied in patients, involves the lab engineering of natural killer (NK) cells — white blood cells that destroy tumor cells — to more effectively battle hepatocellular carcinoma (HCC), one of the most treatment-resistant types of solid tumor.
Genetically modified NK-cell therapy doesn't require personalization like chimeric antigen receptor (CAR)-expressing T-cell therapy — a relatively new, personalized form of immunotherapy. That means an NK-cell therapy could be mass produced and shelf-ready for patients, who could begin therapy without delay, their new research shows.
“To some extent all tumor cells — perhaps hepatocellular carcinoma more so — inhibit immune cells that try to kill them,” said UC San Diego School of Medicine Professor Dan Kaufman, M.D., Ph.D., lead author on the study, director of the Sanford Advanced Therapy Center at the university’s Sanford Stem Cell Institute and Moores Cancer Center member.
“This is one key reason why some immunotherapies like CAR T cells have been less successful for solid tumors than for blood cancers — the immunosuppressive tumor microenvironment.”
Kaufman and his team produced stem cell-derived NK cells in which the receptor for transforming growth factor beta (TGF-β) — a protein that impairs immune function — was disabled. HCC tumors and the liver in general contain copious amounts of the substance, which both inhibits the immune cell activity and allows cancer to proliferate.
They found that typical NK cells without the disabled receptor, like CAR T cells, were not very effective in battling the cancer. “These are pretty resistant tumors — when we put them in mice, they grow and kill the mice,” he said. The five-year survival rate for HCC in humans is less than 20 percent.
When researchers tested the modified NK cells against the cancer, however, “we got very good anti-tumor activity and significantly prolonged survival,” he noted.
Reference: Thangaraj JL, Coffey M, Lopez E, Kaufman DS. Disruption of TGF-β signaling pathway is required to mediate effective killing of hepatocellular carcinoma by human iPSC-derived NK cells. Cell Stem Cell. 2024. doi: 10.1016/j.stem.2024.06.009
Delivery of stem cell therapy for Parkinson’s safe in primate trial
Delivery of stem cell therapy for Parkinson’s safe in primate trial
Transplant approach to delivering therapy feasible, study says
by Lindsey Shapiro, PhD | August 2, 2024
A transplant approach used to deliver ANPD001, an investigational stem cell therapy for Parkinson’s disease, to the brain was found to be safe and feasible in non-human primates.
Data from these preclinical studies supported developer Aspen Neuroscience’s successful application to U.S. regulators seeking to launch a first-in-human study of the experimental treatment. The Phase 1/2a ASPIRO trial (NCT06344026) trial, enrolling by invitation only, is now testing ANPD001 in people with moderate to severe Parkinson’s disease.
“The study was an important step in our work to bring the promise of a cell-replacement therapy to people with Parkinson’s disease,” Andrés Bratt-Leal, PhD, co-founder and senior vice president of research and development at Aspen and one of the study’s authors, said in a company press release. “The results were instrumental in opening our first-in-human trial and informing how we deliver patients’ own cells.”
The Aspen-funded study, “Preclinical evaluation of transaxial intraputaminal trajectory for enhanced distribution of grafted cells in Parkinson’s disease,” was published in the Journal of Neurosurgery.
The symptoms of Parkinson’s disease are caused by the progressive loss of dopaminergic neurons, or nerve cells that produce the major brain signaling chemical dopamine. ANPD001 aims to replace these lost dopaminergic neurons as a way of slowing or stopping progression of the neurodegenerative disease.
August 2, 2024 |
Adaptimmune’s Tecelra Becomes First FDA-Approved Engineered Cell Therapy for Solid Tumors
Approved under the regulator’s accelerated pathway, Tecelra is also the first new synovial sarcoma therapy in more than a decade, according to Adaptimmune Therapeutics
Adaptimmune Therapeutics announced late Thursday that the FDA has approved afamitresgene autoleucel—now to carry the brand name Tecelra—for the treatment of unresectable or metastatic synovial sarcoma.
Tecelra is the first engineered T cell therapy for solid tumor cancers in the U.S. and is the first new therapeutic option for synovial sarcoma in a more than 10 years, according to Adaptimmune. Tecelra was approved under the FDA’s accelerated pathway and Adaptimmune will need to verify its clinical benefit in a confirmatory trial to keep the product on the market.
Designed to be a one-time therapy, Tecelra is an engineered T cell therapy that makes use of a patient’s own immune cells and modifies them to be able to target cancer cells. Tecelra is designed to recognize and attack the melanoma-associated antigen A4 (MAGE-A4) protein, which is crucial to preventing cell cycle arrest and cell death. MAGE-A4 is commonly overexpressed in solid tumors, especially synovial sarcoma.
When activated after binding to MAGE-4, Tecelra induces the proliferation of T cells and secretion of cytokines, leading to the destruction of cancer cells expressing the antigen, according to its label.
Tecelra’s approval is based on data from the SPEARHEAD-1 study, which found an overall response rate of 43% in patients treated with the T-cell therapy. Complete response was reported in 4.5% of patients. Treatment response lasted for at least 12 months in 39% of study participants who were responsive to the therapy.