R&D Innovation
Project Development
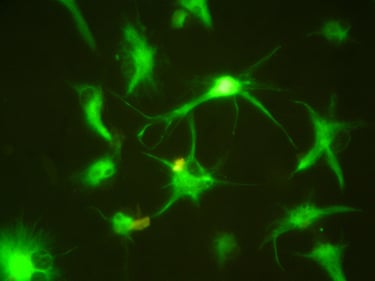
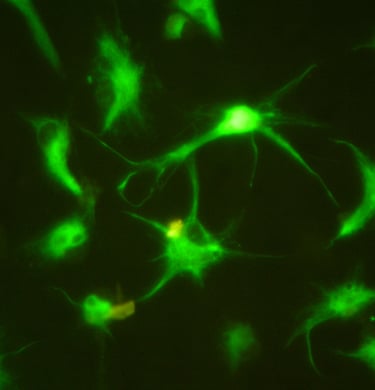
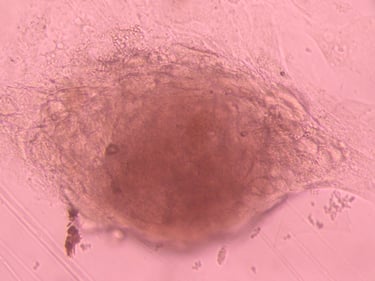
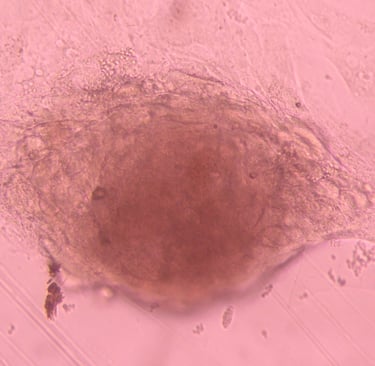
Stem cell Standardisation




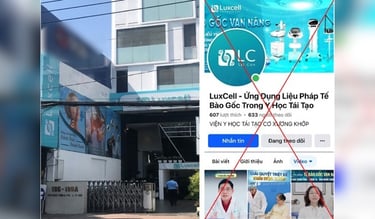
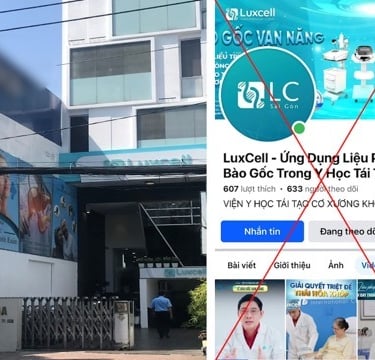






Vietnam, with a population of over 100 million and a rapidly growing economy, is emerging as a vibrant location in the standardized stem cell market in Asia.
In Vietnam, Stem cells and stem cell technology are a field of biotechnology that lags behind developed countries in terms of investment, experts and basic research. However, within the framework of state regulations, clinical research and treatment applications have been carried out in many hospitals and have had demonstrated encouragingpositive results.
The above results have triggered a vibrant development in the application of stem cells outside the legal mainstream. Many companies have entered the Vietnamese market, advertising treatment tourism, sending stem cell samples for preservation in abroad cryobanks. Many private hospitals and clinics quickly opened medical and cosmetic treatment services using stem cells of unproven and illegal origin.
The need to manage the stem cell market through organizing a Network of safe application, using stem cell of identified origin and quality according to international standards has become urgent and has been adopted as a key program of the Ministry of Health.
The stem cell R&D platform project according to international GMP-cGMP standards is an indispensable link of the above program, it meets the immediate needs of the market for stem cell standardization, and is also a premise for updating and developing future technologies.